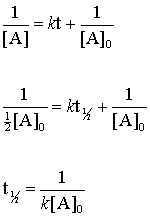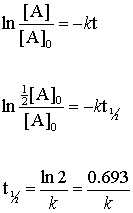half life formula for first order reaction
The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction. A second-order reactions half-life equation is t121kA0 t 1 2 1 k A 0.
Chemical Kinetics Chapter 14 Chemistry The Central Science
Ln 12A0A0 -kt12.
. 2 0693 into the equation results in the expression for the half-life of a first-order reaction. For a zero order reaction Half life decreases with decreasing concentration For a 1st order reaction. For the first order reaction you can plug the definition of the half life into the concentration-time reaction to obtain a neat relationship.
Under given conditions the half-life of a first-order reaction is constant ie the change is zero. T12ln2k t 1 2 l n 2 k is the half-life equation for a first-order reaction. T ½ A o 2k For a first order reaction A products rate kA.
T120693k where t12 is the half-life in seconds s and k is the rate constant in inverse seconds s1. 2 k t 1 2. For a zero order reaction A products rate k.
Half Life of First Order Reactions First-Order Reactions We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. How do the half-lives of first-order and second order reactions differ. The half-life of a first-order reaction is given as t 12 0693k.
Determining a half life. The half-life of a first-order reaction does not depend upon the concentration of the reactant. Half-life equation for first-order reactions.
The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value. Hence we can say it depends linearly on the concentration of one reactant. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions.
For a first-order reaction. The half-life of a second-order reaction is given by the formula 1kR 0. FraclnA_0A_tkt tfraclnA_0A_ttimesfrac1k.
The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02. For a half-life tt_frac12 and A_tfrac12A_0 Substituting into the integrated rate law. Hence the half-life period of a reaction of the first order is independent of the initial concentration of reactant but depends upon only the rate constant of the first order.
T 12 0693k. The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure. Substituting the values in the expression for the rate constant of half-life first-order reaction the.
The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant. 453 t 1 2 0693 k. Notice that the half life does not depend on the reactant concentration.
Now a first-order reaction is characterized by the fact that the rate of the reaction depends linearly on the concentration of one reactant. Where The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R 0 in molL. The half-life of a first-order reaction is given as t 12 0693k.
Frac12A_0 k t_frac12 A_0 And rearranging. Graphical relations and half lives. In other words the half-life of a reaction is calculated when concentration A reduces to A2 in a given period.
Get the free Half Life Calculator first order reaction widget for your website blog Wordpress Blogger or iGoogle. Ln 2 0693 kt12. Find more Chemistry widgets in WolframAlpha.
Equations for Half Lives. It is a constant and related to the rate constant for the reaction. A A 0 e k t AA_0 e-kt A A 0 e k t.
For a first-order reaction the half. How do the half-lives of first-order and second order reactions differ. It is called the half-life of a reaction.
T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. Thus for a first-order reaction each successive half-life is the same length of time as shown in. Converting a half life to a rate constant.
The half-life t_12 is a timescale on which the initial population is decreased by half of its original value represented by the following equation. A dfrac12 A_o After a period of one half-life t t_12 and we can write. T ½ 1 k A o Top.
What is the half-life of a first-order reaction with a rate constant of 870104 s1. The half-life of a chemical reaction regardless of its order is simply the time needed for half of an initial concentration of a reactant to be consumed by the reaction. Half-lives of first order reactions.
For a zero order reaction. Using the half-life equation derived from the concentration-time equation as shown in example 1 we can solve for the initial concentration of reactant. T_12 frac1A_0k.
What is the rate constant of a first-order reaction that takes 533 seconds for. Half-Life of a First-Order Reaction Recall that for a first-order reaction the integrated rate law is given by.

Determine The Half Life Of A First Order Reaction Youtube

First Order Reaction Definition Example Half Life Period Chemist Notes

First Order Reaction Definition Examples And Equations
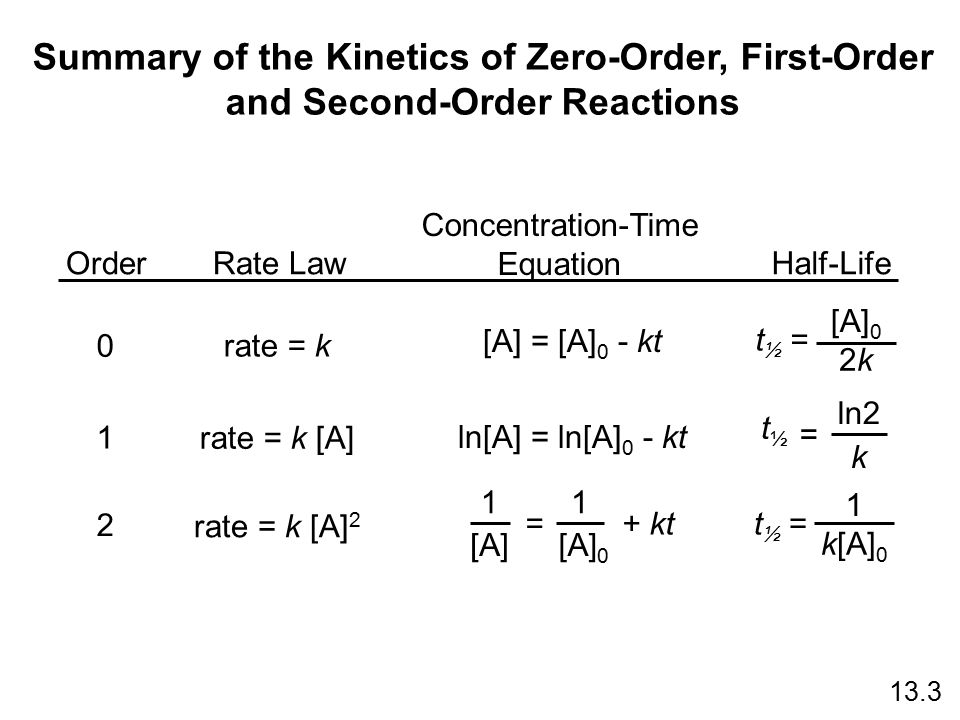
Summary Of The Kinetics Of Zero Order First Order Ppt Download

Organic Chemistry Half Life And Shelf Life Of Second Order Reaction Chemistry Stack Exchange

Zero Order Reactions Video Kinetics Khan Academy

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200s 1 B 2 Mi N 1 C 4years 1
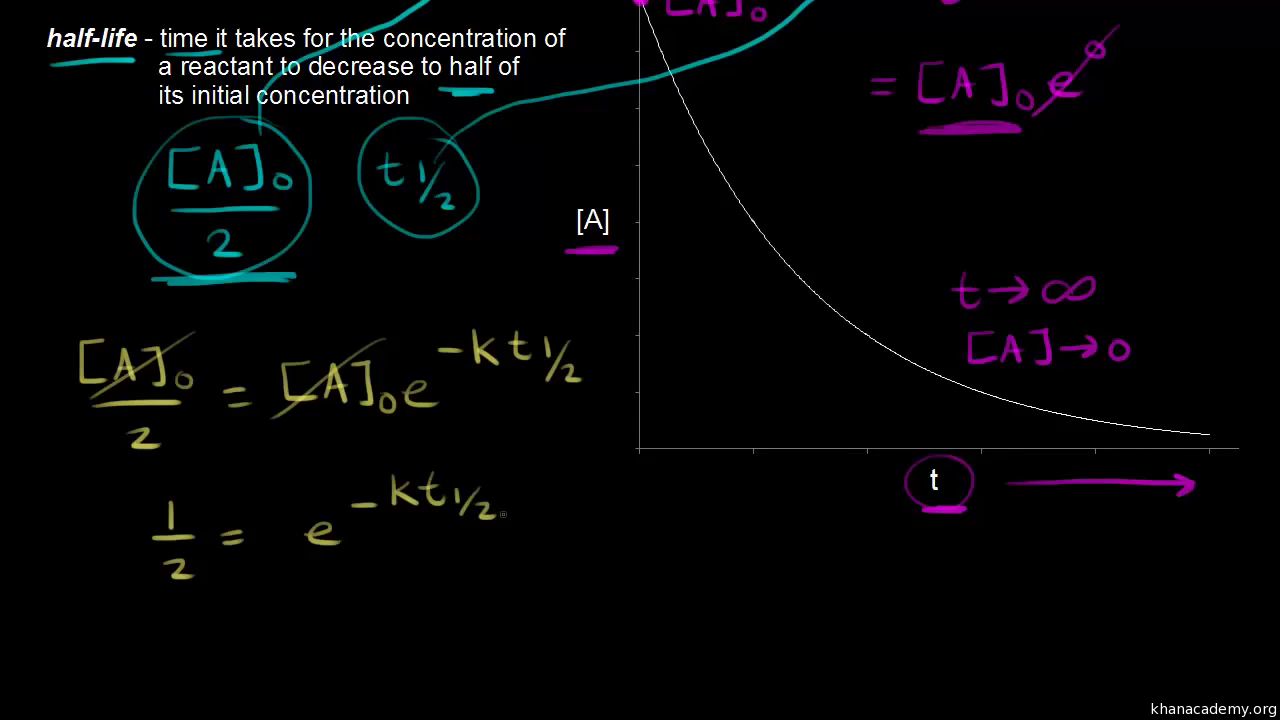
Half Life Of A First Order Reaction Video Khan Academy

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Half Life Of A First Order Reaction Video Khan Academy